|
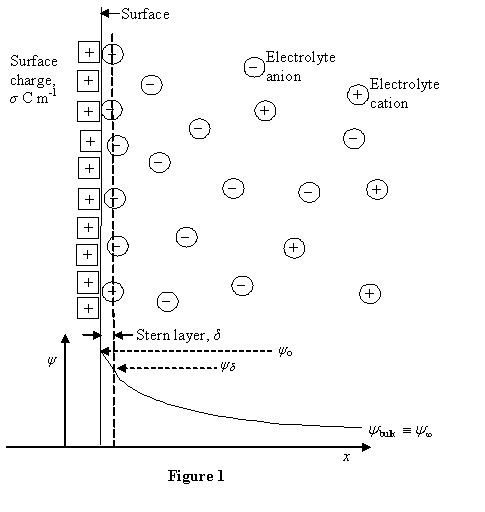

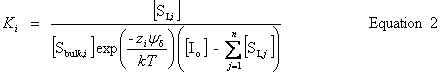
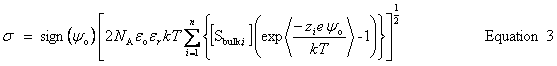
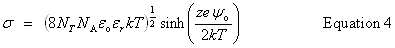

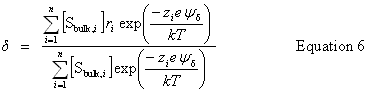


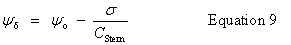
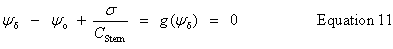

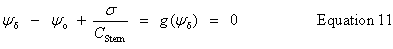

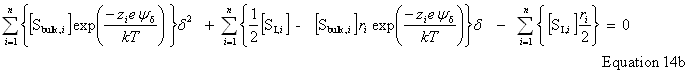

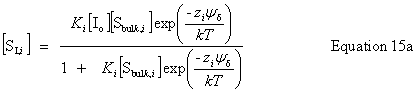
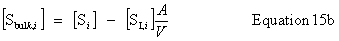
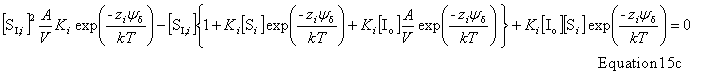
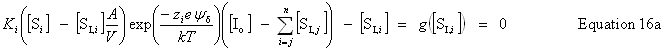
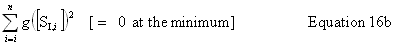
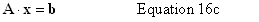
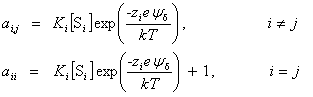



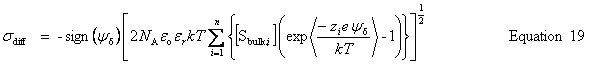
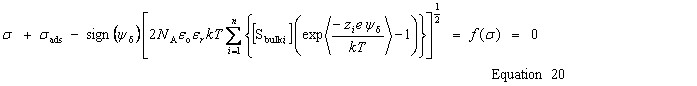
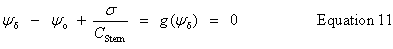



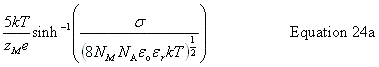

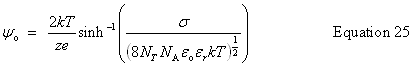
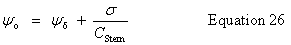

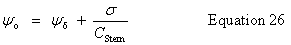

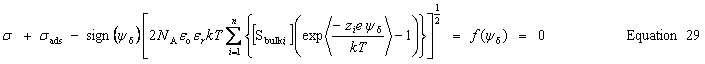
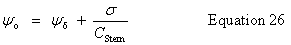

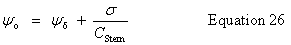
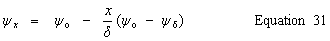

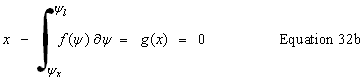
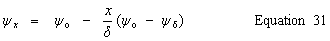

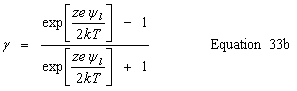
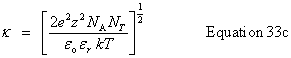
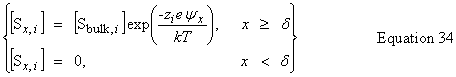


| Constructor |
public GouyChapmanStern() | |
| Stern modification options |
include Stern modification (default option) |
public void includeStern() |
| ignore Stern modification |
public void ignoreStern() | |
|
Ionic radii options |
hydrated radii (default option) |
public void setHydratedRadii() |
| bare radii |
public void setBareRadii() | |
| Enter an ion |
public void setIon(String ion, double concn, double radius, int charge, double assocK) public void setIon(String ion, double concn, double radius, int charge) public void setIon(String ion, double concn, double assocK) public void setIon(String ion, double concn) | |
| Enter the adsorption site density |
public void setSurfaceSiteDensity(double density) | |
| Enter the surface charge density, σ |
public void setSurfaceChargeDensity(double chargeDensity) public void setSurfaceCharge(double charge, double area) public void setSurfaceCharge(double charge) | |
| Enter the surface area |
public void setSurfaceArea(double area) | |
| Enter the electrolyte volume |
public void setVolume(double volume) | |
| Enter the surface potential, ψo |
public void setSurfacePotential(double surfacePotential) | |
| Enter relative electrical permittivities |
public void setRelPerm(double relPerm, double relPermStern) public void setRelPerm(double relPerm) | |
| Enter the temperature |
public void setTemp(double temp) | |
| Get the calculated charge densities | Surface charge density, σ |
public double getSurfaceChargeDensity() | Surface charge |
public double getSurfaceCharge() |
Diffuse layer charge density |
public double getDiffuseChargeDensity() |
Adsorbed ion charge density |
public double getAdsorbedChargeDensity() |
| Get the calculated potentials | Surface Potential potential, ψo |
public double getSurfacePotential() |
| Stern layer - diffuse layer interface potential, ψδ |
public double getDiffuseLayerPotential() | |
| Stern layer potential difference, ψo - ψδ |
public double getSternLayerPotential() | |
| Potential at a distance, x, from the surface |
public double getPotentialAtX() | |
| Get the concentrations | Equilibrium bulk concentrations |
public double[] getBulkConcn() |
| Adsorbed ion surface concentrations |
public double[] getSiteConcns() | |
| Initial concentrations |
public double[] getInitConcns() | |
| Concentrations at a distance, x, from the surface |
public double[] getConcnsAtX() | |
| Initial ionic strength |
public double getIonicStrength() | |
| Get the calculated lengths | Debye length |
public double getDebyeLength() |
| Stern layer thickness |
public double getSternThickness() | |
| Get the calculated capacitances | Total interface capacitance |
public double getTotalCapacitance() public double getTotalCapPerSquareMetre() |
| Diffuse double layer capacitance |
public double getDiffuseLayerCapacitance() public double getDiffuseLayerCapPerSquareMetre() | |
| Stern layer capacitance |
public double getSternCapacitance() public double getSternCapPerSquareMetre() | |